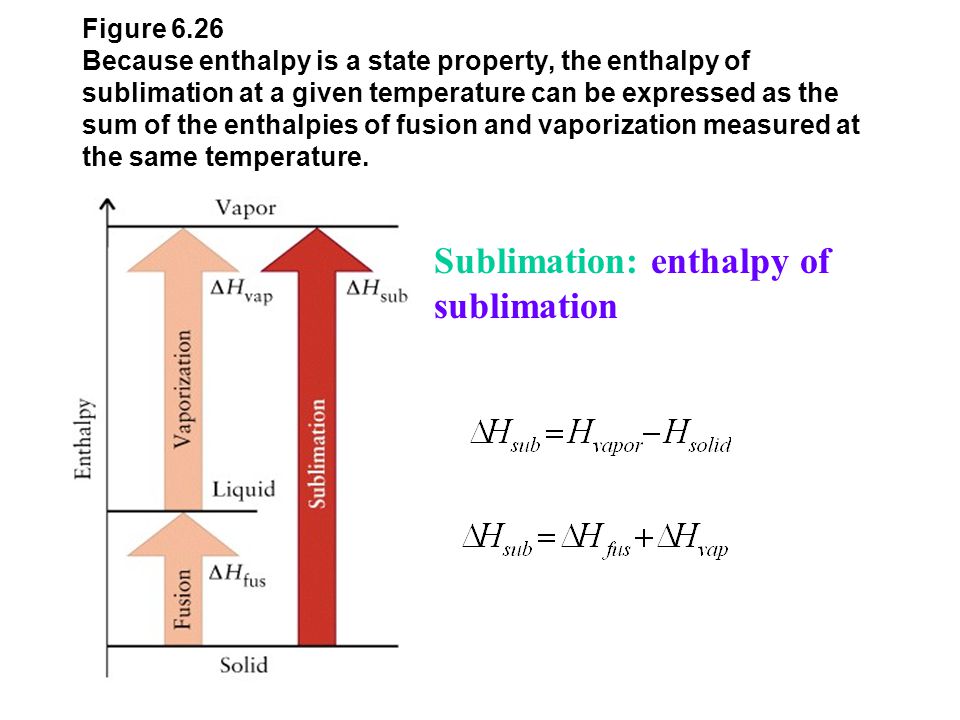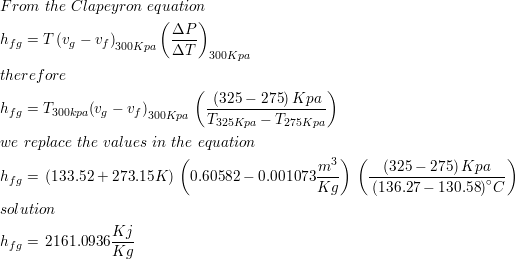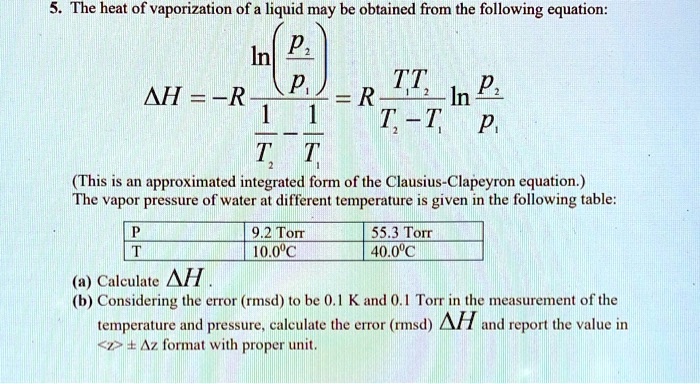
SOLVED: The heat of vaporization of a liquid may be obtained from the following equation: ΔH = -R * ln(P2/P1) * T1 / (T2 - T1) (This is an approximated integrated form

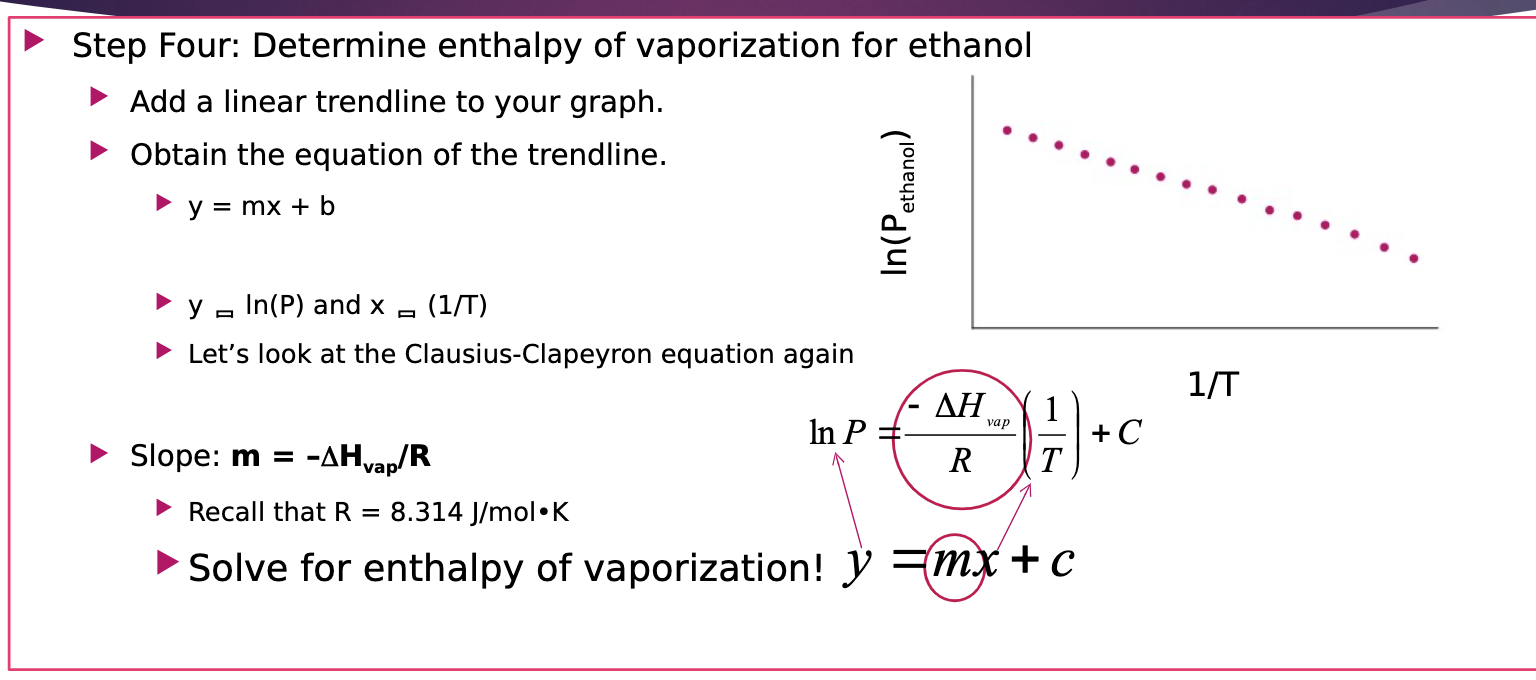
SOLVED: The linear relationship between vapor pressure and the absolute Clausius-Clapeyron equation: temperature (T) in Kelvin, vapor pressure (P), heat of vaporization (Hvap), and the positive constant (C). The equation of a


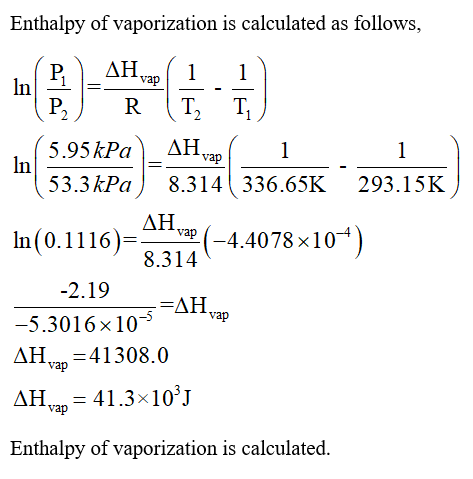





-438.png)